Background: Patients with sickle cell disease (SCD) experience varied salient symptoms that impact their health-related quality of life, which incorporates illness severity, their level of distress and other aspects of impairment, and the impact of the illness on functional status. SCD symptoms may begin at an early age, and their severity varies over time and between patients. Although detection of changes in patients' self-assessed symptoms may be obfuscated by their lifelong symptoms, treating physicians with SCD expertise may be able to detect changes in a patient's status given their experience in treating the diverse symptoms across multiple patients. We evaluated the longitudinal impact of voxelotor (Oxbryta®) in the HOPE study using the single-item Clinical Global Impression of Change (CGI-C), a validated clinician-reported outcome analogous to the commonly used Patient Global Impression of Change (PGI-C), which provides expert clinical impressions that transcend symptom checklists.
Methods: In the randomized, placebo-controlled, double-blinded HOPE study, participants with SCD aged 12 to 65 years were randomized to receive voxelotor (1500 mg or 900 mg dose administered orally, once daily) or placebo. Study participants had a hemoglobin (Hb) level 5.5 to 10.5 g/dL at enrollment and 1 to 10 vaso-occlusive crises in the past 12 months at screening. Clinicians were blinded to treatment assignment and to all central laboratory measures (eg, hematological parameters) throughout the 72-week study period. Clinicians rated the CGI-C at 24 and 72 weeks from baseline. CGI-C ratings used a 7-point scale ("very much improved," "moderately improved," "minimally improved," "no change," "minimally worse," "moderately worse," and "very much worse"). CGI-C outcomes were stratified by baseline Hb levels and baseline markers of hemolysis to identify measures predictive of assessment outcomes.
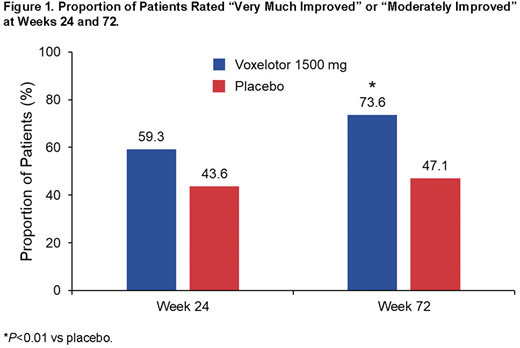
Results: Trends of improvement from baseline in overall clinical status with voxelotor 1500 mg, as assessed by CGI-C scores, were observed as early as week 24. At week 72, patients receiving voxelotor 1500 mg were more often rated by the clinician as "very much improved" or "moderately improved" (74%; 39 of 53) compared with those who received placebo (47%; 24 of 51) (P<0.01, Figure 1). CGI-C score improvement was consistently greater in participants treated with voxelotor compared with placebo regardless of baseline Hb. Greater proportions of participants were rated "very much improved" or "moderately improved" with voxelotor 1500 mg than placebo regardless of baseline hemolysis markers (indirect bilirubin, percentage reticulocytes, absolute reticulocyte count).
Conclusions: Treatment with voxelotor 1500 mg resulted in improved clinician-reported patient outcomes, as assessed using the CGI-C. Greater proportions of participants were rated "very much improved" or "moderately improved" regardless of baseline Hb levels and baseline markers of hemolysis. The CGI provides an overall clinician-determined summary measure that takes all available information into account, including the patient's history, psychosocial circumstances, symptoms, and behavior, as well as the impact of symptoms on the patient's ability to function. Therefore, a tool that provides a holistic assessment of a patient's well-being may be complementary in capturing changes in a patient's status, considering the substantial interpatient and intrapatient variability in SCD symptomatology. CGI-C assessments are being included in ongoing studies of voxelotor.
Smith:Emmaeus Pharmaceuticals, Inc.: Consultancy; Novartis, Inc.: Consultancy, Other: Investigator, Research Funding; Global Blood Therapeutics, Inc.: Consultancy, Research Funding; Shire, Inc.: Other: Investigator, Research Funding; NHLBI: Research Funding; Patient-Centered Outcomes Research Institute: Other: Investigator, Research Funding; Health Resources and Services Administration: Other: Investigator, Research Funding; Incyte: Other: Investigator; Pfizer: Consultancy; Ironwood: Consultancy; Novo Nordisk: Consultancy; Imara: Research Funding; Shire: Research Funding; GlycoMimetics, Inc.: Consultancy. Hankins:American Society of Pediatric Hematology/Oncology: Honoraria; UptoDate: Consultancy; Novartis: Research Funding; MJH Life Sciences: Consultancy, Patents & Royalties; LINKS Incorporate Foundation: Research Funding; National Heart, Lung, and Blood Institute: Honoraria, Research Funding; Global Blood Therapeutics: Consultancy, Research Funding. Abboud:Novartis: Consultancy, Honoraria, Research Funding; Global Blood Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Crispr Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Other: Travel support; Eli Lilly: Research Funding; Modus Pharmaceuticals: Research Funding. Cong:Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. Sorof:Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. Gray:Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. Hoppe:Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. Telfer:Global Blood Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Terumo: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Honoraria, Research Funding; ApoPharma: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal